A cell-compatible methyl cellulose for biotech applications
METHONOVA™ is the latest addition to our renowned NovaMatrix® range, a trusted portfolio of biopolymers tailored for use in the biotechnology industry. Our METHONOVA™ methyl cellulose builds on decades of polymer science expertise and meets the exacting standards of NovaMatrix®, undergoing elaborate quality testing for bioburden and elemental impurities.
METHONOVA™ has a low fiber content, easing sterile filtration to create a highly purified product suitable for use in cell culture. Cytotoxicity testing ensures METHONOVA™ is compatible with cells. Our cutting-edge solution is responsibly sourced, and adheres to stringent product specifications, making it a preferred choice for high-quality biotechnology and bioprocessing applications.
Chemistry
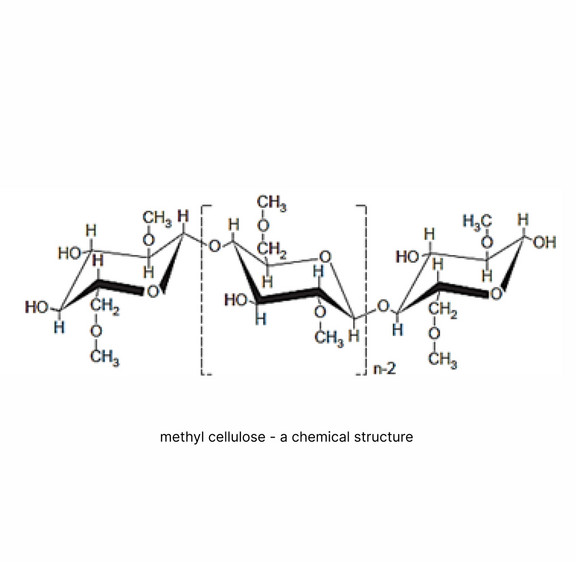
METHONOVA™ is a plant-based, water-soluble biopolymer derived from cellulose, the most abundant polymer in nature. METHONOVA™ methyl cellulose is a natural carbohydrate composed of repeating anhydroglucose units. During manufacture, cellulose fibers are treated with a caustic solution, followed by methyl chloride treatment, yielding the methyl ether of cellulose. The fibrous reaction product is purified and ground to a fine powder.
Product benefits

- Ease of sterile filtration
- Cell compatibility, verified via cytotoxicity test to mammalian cells
- Stringent quality standards
- Outstanding viscosity stability during long-term storage
- Water-solubility
- Reverse thermo-gelation properties
- Xeno-free formulation
Key applications

METHONOVA™ methyl cellulose is suitable for use in:
- Cell culture assays
- Stem cell protection
- Cryopreservation and cell media
- 3D bioprinting
Specifications and Quality
Product | Apparent Viscosity (2%, 20C) | Quality testing* |
|---|---|---|
METHONOVA™ | 13-17 mPa*s | Cell compatibility (cytotoxicity to mammalian cells) Elemental impurities Bioburden Fiber content |
* Examples only and not representative of a complete list of all quality tests
Regulatory
METHONOVA™ methyl cellulose is manufactured under excipient cGMP requirements. It is certified to meet the respective monographs: USP (United States Pharmacopeia), EP/PhEur (European Pharmacopeia) and JP (Japanese Pharmacopeia).
Do you have questions regarding METHONOVA™ or are you interested in buying the product? Get in touch!
Do you have questions regarding METHONOVA™ or are you interested in buying the product? Get in touch!
We can help you along your product development process. Contact us to start your journey to success.